Eu2P MASTER FIRST YEAR PROGRAMME
| 2026 | 2027 |
| Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | Jul |
| 01 | |
01 | |
01 | |
01 | |
01 | | |
01 | | |
01 | | |
01 | | |
01 | | |
01 | | |
01 | |
| 02 | |
02 | |
02 | |
02 | |
02 | | |
02 | | |
02 | | |
02 | | |
02 | | |
02 | | |
02 | |
| 03 | |
03 | |
03 | |
03 | |
03 | | |
03 | | |
03 | | |
03 | | |
03 | | |
03 | | |
03 | |
| 04 | |
04 | |
04 | |
04 | |
04 | | |
04 | | |
04 | | |
04 | | |
04 | | |
04 | | |
04 | |
| 05 | |
05 | |
05 | |
05 | |
05 | | |
05 | | |
05 | | |
05 | | |
05 | | |
05 | | |
05 | |
| 06 | |
06 | |
06 | |
06 | |
06 | | |
06 | | |
06 | | |
06 | | |
06 | | |
06 | | |
06 | |
| 07 | |
07 | |
07 | |
07 | |
07 | | |
07 | | |
07 | | |
07 | | |
07 | | |
07 | | |
07 | |
| 08 | |
08 | |
08 | |
08 | |
08 | | |
08 | | |
08 | | |
08 | | |
08 | | |
08 | | |
08 | |
| 09 | |
09 | |
09 | |
09 | |
09 | | |
09 | | |
09 | | |
09 | | |
09 | | |
09 | | |
09 | |
| 10 | |
10 | |
10 | |
10 | |
10 | | |
10 | | |
10 | | |
10 | | |
10 | | |
10 | | |
10 | |
| 11 | |
11 | |
11 | |
11 | |
11 | | |
11 | | |
11 | | |
11 | | |
11 | | |
11 | | |
11 | |
| 12 | |
12 | |
12 | |
12 | |
12 | | |
12 | | |
12 | | |
12 | | |
12 | | |
12 | | |
12 | |
| 13 | |
13 | |
13 | |
13 | |
13 | | |
13 | | |
13 | | |
13 | | |
13 | | |
13 | | |
13 | |
| 14 | |
14 | |
14 | |
14 | |
14 | | |
14 | | |
14 | | |
14 | | |
14 | | |
14 | | |
14 | |
| 15 | |
15 | |
15 | |
15 | |
15 | | |
15 | | |
15 | | |
15 | | |
15 | | |
15 | | |
15 | |
| 16 | |
16 | |
16 | |
16 | |
16 | | |
16 | | |
16 | | |
16 | | |
16 | | |
16 | | |
16 | |
| 17 | |
17 | |
17 | |
17 | |
17 | | |
17 | | |
17 | | |
17 | | |
17 | | |
17 | | |
17 | |
| 18 | |
18 | |
18 | |
18 | |
18 | | |
18 | | |
18 | | |
18 | | |
18 | | |
18 | | |
18 | |
| 19 | |
19 | |
19 | |
19 | |
19 | | |
19 | | |
19 | | |
19 | | |
19 | | |
19 | | |
19 | |
| 20 | |
20 | |
20 | |
20 | |
20 | | |
20 | | |
20 | | |
20 | | |
20 | | |
20 | | |
20 | |
| 21 | |
21 | |
21 | |
21 | |
21 | | |
21 | | |
21 | | |
21 | | |
21 | | |
21 | | |
21 | |
| 22 | |
22 | |
22 | |
22 | |
22 | | |
22 | | |
22 | | |
22 | | |
22 | | |
22 | | |
22 | |
| 23 | |
23 | |
23 | |
23 | |
23 | | |
23 | | |
23 | | |
23 | | |
23 | | |
23 | | |
23 | |
| 24 | |
24 | |
24 | |
24 | |
24 | | |
24 | | |
24 | | |
24 | | |
24 | | |
24 | | |
24 | |
| 25 | |
25 | |
25 | |
25 | |
25 | | |
25 | | |
25 | | |
25 | | |
25 | | |
25 | | |
25 | |
| 26 | |
26 | |
26 | |
26 | |
26 | | |
26 | | |
26 | | |
26 | | |
26 | | |
26 | | |
26 | |
| 27 | |
27 | |
27 | |
27 | |
27 | | |
27 | | |
27 | | |
27 | | |
27 | | |
27 | | |
27 | |
| 28 | |
28 | |
28 | |
28 | |
28 | | |
28 | | |
28 | | |
28 | | |
28 | | |
28 | | |
28 | |
| 29 | |
29 | |
29 | |
29 | |
29 | | |
| | |
29 | | |
29 | | |
29 | | |
29 | | |
29 | |
| 30 | |
30 | |
30 | |
30 | |
30 | | |
| | |
30 | | |
30 | | |
30 | | |
30 | | |
30 | |
| | |
31 | |
| |
31 | |
31 | | |
| | |
31 | | |
| | |
31 | | |
| | |
31 | |
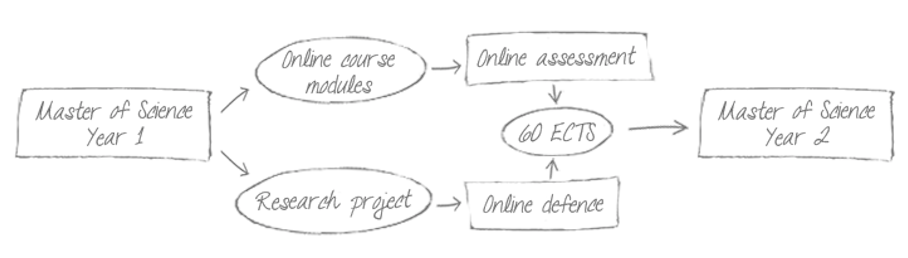
Fresher's week session (face-to-face)
Online course period
Schedule for online meeting sessions, excercice deadline, and resource availability will be provided by the trainers' team
Online Assessment period
Details on the exam organisation are provided by the trainers' team
Research project period
Online Research project defence
BASICS IN EPIDEMIOLOGY
OBJECTIVES
To understand the basic concepts and principles in epidemiology
To become familiar with the epidemiological tools that could be used in pharmacovigilance and pharmacoepidemiology
To understand the specificity of epidemiological tools applied to pharmacovigilance and pharmacoepidemiology
MODULE PARTS
- Descriptive epidemiology (person, place and time)
- Observational studies in pharmacoepidemiology
- Biases in pharmacoepidemiology studies
- Causality criteria, evidence level and critical reading
TRAINER TEAM
Coordinator: Prof Antoine Pariente (Université de Bordeaux)
Experts: Prof Francesco Salvo, Adeline Degremont (Université de Bordeaux)
BASICS IN STATISTICS
OBJECTIVES
To understand applications of probability in statistics
To understand how to organize and describe epidemiological data
To understand how to perform data estimations, comparisons and predictions
To understand how to estimate the sample size and power of epidemiology studies
To introduce the principles of multivariable comparisons
MODULE PARTS
- Introduction to statistics and probability
- Estimates
- Statistical testing
- Introduction to multivariable linear regression
TRAINER TEAM
Coordinator: Dr Karine Palin (Université de Bordeaux)
Experts: Prof Francesco Salvo, Frederic Daoud (Université de Bordeaux)
BASICS IN CLINICAL PHARMACOLOGY
OBJECTIVES
To understand the role of medicines in therapeutics, health services and society
To become familiar with the life cycle of medicines and the regulatory aspects of medicines market
To review the clinical and pharmacological basis of prescribing
To understand the need to evaluate the effects of medicines from an epidemiological perspective
To understand the relevance of unbiased information to ensure the appropriate use of medicines
To discuss the need to monitor medicines prescribing process
MODULE PARTS
- Clinical pharmacology. Aims and uses in the therapeutics.
- Clinical pharmacology and epidemiological evaluation of medicine effects
- The randomized clinical trials (RCT) as a method for assess the efficacy of medicines
- Basics for RCT appraisal: extrapolation of RCTs results to clinical practice.
TRAINER TEAM
Coordinator: Dr Antònia Agustí (Universitat Autònoma de Barcelona)
Experts: Janice Fuller (Amgen NV), Prof Joan-Ramon Laporte, Josep Maria Castel, Dr Eduard Diogène, Lina Camacho (Universitat Autònoma de Barcelona), Prof Albert Figueras (World Health Organization), Dr Ulf Bergman (Karolinska Institutet)
PRINCIPLES OF PHARMACOVIGILANCE
OBJECTIVES
To enable you to develop an understanding of the principles of pharmacovigilance from the development of the science to its place in pre and post-authorisation environment and the roles of various stakeholders within pharmacovigilance.
MODULE PARTS
- Introduction to pharmacovigilance principles.
- Safety evaluation during a products lifecycle.
- Labelling and risk management.
TRAINER TEAM
Coordinator: Sue Rees (University of Hertfordshire)
Experts: Dr Sherael Webley, Dr Christopher Benham, Jabeen Ahmadi (University of Hertfordshire), Wendy Brewster, Steve Hobbiger (GlaxoSmithKline Research and Development Ltd), Dr David Lewis (Novartis Pharma AG), Shelley Ghandi (NDA Regulatory Science), Dr James Whitehead (AstraZeneca AB)
BASICS IN PHARMACOEPIDEMIOLOGY
OBJECTIVES
To understand how to evaluate the effects of medicines and therapeutic interventions both during drug development and clinical use
To know the main methodological and ethical characteristics and limitations of randomized clinical trials
To understand the differences between efficacy and effectiveness
To know the basic concepts and classification of the pharmacoepidemiological studies designed to evaluate therapeutic interventions
To identify information gaps in selected therapeutic areas (e.g. cardiovascular, psychiatry, diabetes care)
MODULE PARTS
- Patients, medicines and prescribing in society. Life cycle of medicines.
- The knowledge-building process of the effects and adverse effects of medicines during their development and clinical use.
- Overview of studies to detect and to evaluate the risk associated with therapeutic interventions. Scope, uses and limitations.
- The need to monitor the medicines prescribing process.
- Introduction to the study of the use of medicines in clinical practice.
TRAINER TEAM
Coordinator: Dr Imma Danés (Universitat Autònoma de Barcelona)
Experts: Janice Fuller (Amgen NV), Prof Joan-Ramon Laporte, Josep Maria Castel, Dr Eduard Diogène, Dr Antònia Agustí, Dr Xavier Vidal, Melani Nuñez Montero (Universitat Autònoma de Barcelona), Prof Albert Figueras (World Health Organization), Noah Jamie Robinson (F. Hoffmann-La Roche Ltd), Dr Ulf Bergman (Karolinska Institutet), Lucia Bellas Fernández (European Health Data and Evidence Network)
BASICS IN MEDICINES RISK COMMUNICATION
OBJECTIVES
To know the basic principles of communication and to gain an insight into the characteristics of effective communication
To face to the complexity communication on risk for health
To know the evolution of medicine risk communication
MODULE PARTS
- Which knowledge and skills are useful for communication?
- Concrete examples of communication: what do we retain?
- Why communicate? Nature and importance of communication. Personal and social dimension of communication
- Face to face between doctor and patient. How to find the right word?
TRAINER TEAM
Coordinator: Prof Annalisa Capuano (Università degli Studi della Campania Luigi Vanvitelli)
Experts: Christa Naboulet, Dr Marie-Pierre Barrouillet (Université de Bordeaux), Bruce Hugman (Independent), Dr Cristina Scavone, Dr Annamaria Mascolo, Prof Liberata Sportiello, Dr Cecilia Cagnotta, Dr Antonietta Anatriello (Università degli Studi della Campania Luigi Vanvitelli)
VALORISATION AND CRITICAL APPRAISAL IN RESEARCH
OBJECTIVES
To improve your scientific communication skills such as:
-
Redaction of scientific original papers and reports
-
Realization of a scientific poster
-
Preparation of a scientific oral communication
To become familiar with the institutions and data sources in Health Sciences
To become familiar with the research funding
MODULE PARTS
- Critical reading of scientific literature: clinical trials and observational studies
- Principles of the redaction of scientific papers and reports
- Introduction to scientific watch: principles and techniques
- Principles of scientific posters and oral communication
- Presentation of the main institutions and data sources in Health Sciences
- Principles for research funding
TRAINER TEAM
Coordinator: Dr Karine Palin (Université de Bordeaux)
Experts: Christa Naboulet, Stéphane Liège, Prof Francesco Salvo (Université de Bordeaux), Dr Yannick Arimone (Agence Nationale pour la gestion des Déchets RAdioactifs)
ANALYSIS AND SYNTHESIS OF HEALTH DATA
OBJECTIVES
Synthesize in a consistent manner available information related to a pharmacoepidemiological problem from different data sources.
TRAINER TEAM
Coordinator: Prof Francesco Salvo (Université de Bordeaux)
Experts: Prof Antoine Pariente (Université de Bordeaux)
PARTNERS' SAVING
Eu2P programme is made by a Public-Private consortium.
Each Eu2P partner benefits from a special price on Eu2P tuition fees for their students or employees!
| Saving for ... |
Student
in a Eu2P University |
Employee
in a Eu2P agency, company or affiliate |
| Short Courses |
- 40% |
- 40% |
| Certificate fees |
- 30% |
- 30% |
| Master year fees |
- 20% |
- 20% |
| PhD year fees |
0% |
0% |
REWARD PROGRAMME
Gain up-to 20% of savings when studying with Eu2P!
Eu2P gives you reward points each time you register to any Eu2P training programmes : Master, Certificate and Short Course. One point amonts to one euro.
You can redeem these points and get savings on next course tuition fees, only for Certificates and Master.The reward programme does not entitle to savings on Short Courses tuition fees.
MASTER & PhD GRANTS
Eu2P offers a limited number of grants to Master and PhD selected applicants to cover partial Master and PhD tuition fees.
Eu2P grants are awarded by the Eu2P consortium and private organizations on the appraisal of the applicant's status regarding:
- Academic performance
- Professional ambition and environment
- Personal situation
- Any special and financial circumstances that may affect his/her Eu2P training performance
Grant application & appraisal
During on-line Master or PhD application, you can confirm that you wish to apply to a grant. Following your successful Master or PhD selection, you must provide a statement regarding the most relevant reasons to support your grant request:
- Certified true copies of your official academic transcripts not already included in your online application
- A brief statement regarding your professional ambition and environment/location
- Statement of proof of special personal or professional circumstances
The application procedure and the selection process are the same for all, regardless of whether you come from Eu2P partners or not, from European or third countries.
All grant applications are ranked by the Eu2P Board. You are warned about your grant application status by e-mail.

 LOG IN OR SIGN UP TO "MY Eu2P"
LOG IN OR SIGN UP TO "MY Eu2P"





